Porifera
Sponges
Dennis Lavrov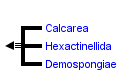

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
Sponges (phylum Porifera) are an exclusively aquatic and, with a few exceptions (Vacelet and Boury-Esnault 1995), a filter-feeding group of animals. The group consists of approximately 15,000 extant species in three distinct groups (Hooper and van Soest 2002):
- the glass sponges (Class Hexactinellida)
- the calcareous sponges (Class Calcarea)
- the demosponges (Class Demospongiae)
Adult sponges can be asymmetrical or radially symmetrical and come in a variety of sizes, colors, and shapes, including arboresecent (tree-like), flabellate (fan-shaped), caliculate (cup shaped), tubular (tube shaped), globular (ball shaped), and amorphous (shapeless) among others. Sponges occupy both freshwater and marine environments, from shallow to abysmal depths, and are common in coral reef, mangroves, and seagrass ecosystems. In some places (e.g., Lake Baikal, Russia) sponges dominate benthic communities and along the pacific cost of North America they form modern sponge reefs.
General Design
The body plan of a sponge is simple (e.g., De Vos et al. 1991): a single outer layer of cells (the pinacoderm) separates the inner cellular region (mesohyl) from the external environment. The pinacoderm lines the internal canals and is eventually replaced by the choanoderm, a layer of characteristic flagellated collar cells (choanocytes) grouped in chambers. Choanocytes make up the principle ‘pump’ and’ filter’ of the system, driving water through the sponge, trapping and phagocytizing suspended bacteria and other particulate food, which is then digested and nutrients distributed among the cells of the mesohyl that facilitate the functions of feeding, respiration and reproduction. The flow of water inside a sponge is unidirectional: the water is drawn in through tiny pores (ostia) in the pinacoderm and exits through one or more larger openings (osculae). The aquiferous system of a sponge is usually supported by a combination of two types of skeletal elements: mineral spicules (either calcareous or siliceous) and special protein fibers (spongin), although either one or both of these elements can be absent.
The 19th-century discovery of a remarkable similarity between porifera-specific choanocytes and free-living choanoflagellates led to a proposition that sponges are the most primitive metazoans, evolved from choanoflagellate-like protist ancestors (Clark 1866; Clark 1868). The ancient origin of sponges is corroborated by the existence of a poriferan fossil record going back to the Early Vendian (~580 Mya) (Li, Chen, and Hua 1998), and by sponge biomarker record going back to the Cryogenian period (~750 Mya) (Love et al. 2009).
Characteristics
Traditionally, sponges have been regarded as a monophyletic group defined by several synapomorphies (Hooper, Van Soest, and Debrenne 2002), including the presence of:
- choanocytes
- an aquiferous system with external pores
- mineral spicules
- high cellular mobility and totipotency
The latter feature is often considered to be the most important (Lévi 1999). Indeed, the sponge body is unique among animals because it continuously remolds itself to fine-tune its filter-feeding system. The constant rearrangement of the body is accomplished by the amoeboid movements of cells inside the sponge and their change from one differentiated form to another.
Although often considered immobile, sponges also display several behavioral patterns (resulting from coordinated movements of cells), including crawling, production of filamentous body extensions and body contractions (Nickel 2004). It is also often mentioned that sponges lack many characteristics associated with other animals, including a mouth, sensory organs, organized tissues and neurons and muscle cells, which are otherwise ubiquitous in Metazoa. It is difficult to say, however, whether the lack of aforementioned features represents a primitive condition of sponges or a secondary loss due to their sedentary and water-filtering lifestyle. Indeed, a recent study has shown that the homoscleromorph sponges possess several characteristics thought to be absent in sponges, including the presence of true epithelia (Boury-Esnault et al. 2003). Another study has found that although sponges do not have neurons, their genome contains most of the components needed to build a post-synaptic protein scaffold that is essential for neural impulse transduction in other animals (Sakarya et al. 2007).
Discussion of Phylogenetic Relationships
Although the presence of three distinct groups among the extant sponges (classes Hexactinellida, Calcarea, and Demospongiae) is largely unchallenged, the evolutionary relationships among them are controversial. Gray (1867) was first to subdivide all sponges into ‘Porifera Silicea’ and ‘Porifera Calcarea’ based on the chemical composition of sponge spicules (silica vs. calcium carbonate), a view still advocated by some scholars (e.g., Böger 1988). More recently, Reid and Reiswig and Mackie (Reiswig and Mackie 1983) subdivided Porifera into ‘Cellularia’ (Demospongiae plus Calcarea) and ‘Symplasma’ (Hexactinellida), based on the syncytial nature of the choanoderm and pinacoderm in glass sponges (Hexactinellida). Unfortunately, neither chemical composition of spicules nor syncitial nature of glass sponges are likely phylogenetically informative. First, a diverse range of siliceous structures is known in different groups of unicellular eukaryotes, including choanoflagellates, the sister group of animals (Preisig 1994). Second, the syncytial tissue in glass sponges appears to be a derived trait within this group because the development of glass sponges starts with a cellular embryo (Leys, Cheung, and Boury-Esnault 2006).
Similarly, molecular studies have so far been also inconclusive in regard to poriferan relationships, although they suggested two interesting alternatives: 1) the paraphyly of sponges (Cavalier-Smith et al. 1996; Collins 1998; Adams, McInerney, and Kelly 1999; Borchiellini et al. 2001; Rokas, Kruger, Carroll 2005; Peterson et al. 2008) and b) the presence of a distinct fourth class of sponges: Homoscleromorpha (Borchiellini et al. 2004; Nichols 2005; Peterson et al. 2008). However, the latest and the largest molecular phylogenetic study supports the traditional view that sponges are monophyletic (Philippe et al., in print).
References
Adams, C. L., J. O. McInerney, and M. Kelly. 1999. Indications of relationships between poriferan classes using full-length 18S rRNA gene sequences. Mem. Queensl. Mus. 44:33-43.
B÷ger, H. 1988. Versuch Řber das phylogenetische System der Porifera. Meyniana 40: 143-154.
Borchiellini, C., C. Chombard, M. Manuel, E. Alivon, J. Vacelet, and N. Boury-Esnault. 2004. Molecular phylogeny of Demospongiae: implications for classification and scenarios of character evolution. Mol. Phylogenet. Evol. 32:823-837.
Borchiellini, C., M. Manuel, E. Alivon, N. Boury-Esnault, J. Vacelet, and Y. Le Parco. 2001. Sponge paraphyly and the origin of Metazoa. J. Evol. Biol. 14:171-179.
Boury-Esnault, N., A. Ereskovsky, C. Bezac, and D. Tokina. 2003. Larval development in the Homoscleromorpha (Porifera, Demospongiae). Invertebr. Biol. 122:187-202.
Cavalier-Smith, T., M. T. E. P. Allsopp, E. E. Chao, N. Boury-Esnault, and J. Vacelet. 1996. Sponge phylogeny, animal monophyly, and the origin of the nervous system: 18S rRNA evidence. Can. J. Zool. 74:2031-2045.
Clark, H. 1866. Note on the infusoria flagellata and the spongiae ciliatae. Am. J. Sci. 1:113-114.
Clark, H. 1868. On the Spongiae ciliatae as infusoria flagellata, or observations on the structure, animality and relationship of Leucosolenia botryoides, Bowerbank. Annu. Mag. Nat. Hist. 4:133-142.
Collins, A. G. 1998. Evaluating multiple alternative hypotheses for the origin of Bilateria: an analysis of 18S rRNA molecular evidence. Proc. Natl. Acad. Sci. U.S.A. 95:15458-15463.
De Vos, L., K. Rutzler, J. V. Boury-Esnault, C. Donadey, and J. Vacelet. 1991. Atlas of sponge morphology = Atlas de morphologie des Úponges. Smithsonian Institution Press, Washington.
Hooper, J. N. A., R. W. M. Van Soest, and F. Debrenne. 2002. Phylum Porifera Grant, 1836. Pp. 9-13 in J. N. A. Hooper, and R. W. M. Van Soest, eds. Systema Porifera: a guide to the classification of sponges. Kluwer Academic/Plenum Publishers, New York.
Hooper, J. N. A., and R. W. M. van Soest. 2002. Systema Porifera: A Guide to the Classification of Sponges. 1810.
LÚvi, C. 1999. Sponge science, from origin to outlook. Memoirs of the Queensland Museum Volume: 44 Part: Year: 1999 44:1-7.
Leys, S. P., E. Cheung, and N. Boury-Esnault. 2006. Embryogenesis in the glass sponge Oopsacas minuta: Formation of syncytia by fusion of blastomeres. Integr. Comp. Biol. 46:185-195.
Li, C. W., J. Y. Chen, and T. E. Hua. 1998. Precambrian sponges with cellular structures. Science 279:879-882.
Love, G. D., E. Grosjean, C. Stalvies et al. 2009. Fossil steroids record the appearance of Demospongiae during the Cryogenian period. Nature 457:718-721.
Nichols, S. A. 2005. An evaluation of support for order-level monophyly and interrelationships within the class Demospongiae using partial data from the large subunit rDNA and cytochrome oxidase subunit I. Mol. Phylogenet. Evol. 34:81-96.
Nickel, M. 2004. Kinetics and rhythm of body contractions in the sponge Tethya wilhelma (Porifera : Demospongiae). JOURNAL OF EXPERIMENTAL BIOLOGY 207:4515-4524.
Peterson, K. J., J. A. Cotton, J. G. Gehling, and D. Pisani. 2008. The Ediacaran emergence of bilaterians: congruence between the genetic and the geological fossil records. Philos Trans R Soc Lond B Biol Sci
Preisig, H. R. 1994. Siliceous structures and silicification in flagellated protists. Protoplasma 181:29-42.
Reiswig, H. M., and G. O. Mackie. 1983. Studies on Hexactinellid Sponges. III. The Taxonomic Status of Hexactinellida Within the Porifera. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 301:419-428.
Rokas, A., D. Kruger, and S. B. Carroll. 2005. Animal evolution and the molecular signature of radiations compressed in time. Science 310:1933-1938.
Sakarya, O., K. A. Armstrong, M. Adamska, M. Adamski, I. F. Wang, B. Tidor, B. M. Degnan, T. H. Oakley, and K. S. Kosik. 2007. A post-synaptic scaffold at the origin of the animal kingdom. PLoS ONE 2:e506.
Vacelet, J., and N. Boury-Esnault. 1995. Carnivorous Sponges. Nature 373:333-335.
Information on the Internet
- Wikipedia: Sponge.
- Porifera. @Palaeos.org.
- FAQ about sponges. Queensland Museum.
- Introduction to Porifera. UCMP Berkeley.
- Phylum Porifera. Animal Diversity Web. University of Michigan Museum of Zoology.
- Khoyatan Sponge Page. Sponges of The Northeast Pacific. Khoyatan Marine Laboratory.
- World Porifera Database.
- Porifera Tree of Life project.
- Sponge Barcoding Project.
- Hexactinellid Reefs.
Title Illustrations

| Scientific Name | Microciona |
|---|---|
| Creator | David Remsen |
| Copyright |
© 1995 Marine Biological Laboratory, Woods Hole

|
| Scientific Name | Chalinula nematifera |
|---|---|
| Location | Borneo, Malaysia |
| Comments | Image of a purple, encrusting sponge (Nara nematifera) on hard reef substrate. |
| Specimen Condition | Live Specimen |
| Source | Sponge |
| Source Collection | Coral Reef Alliance |
| Copyright | © Jeff Dawson |
| Scientific Name | Staurocalyptus sp. |
|---|---|
| Location | California, Davidson Seamount, USA |
| Acknowledgements | NOAA/Monterey Bay Aquarium Research Institute |
| Specimen Condition | Live Specimen |
| Identified By | 2002 May 22 |
| Source | expl0786, Voyage To Inner Space - Exploring the Seas With NOAA Collect |
| Source Collection | NOAA Photo Library |
About This Page
Creation of this page was supported by the NSF grant DEB-0829783 to DL, a part of the ATol: PorToL - The Porifera Tree of Life collaborative research project with Dr. Purushotham Bangalore (University of Alabama at Birmingham), Dr. Allen Collins (Smithsonian Institution National Museum of Natural History), Dr. Cristina Diaz (Smithsonian Institution National Museum of Natural History and Museo Marino Margarita, Venezuela), Dr. April Hill (University of Richmond), Dr. Malcolm Hill (University of Richmond), Dr. John Hooper (Queensland Museum, Australia), Dr. Jose Lopez (Nova Southeastern University), Dr. Kevin Peterson (Dartmouth College), Dr. Shirley Pomponi (Harbor Branch Oceanographic Institution at Florida Atlantic University), Mr. John Reed (Harbor Branch Oceanographic Institution at Florida Atlantic University), Dr. Robert Thacker (University of Alabama at Birmingham), and Dr. Gert Wörheide (University of Göttingen).
Dennis Lavrov

Iowa State University, Ames, Iowa, USA
Correspondence regarding this page should be directed to Dennis Lavrov at
Page copyright © 2009 Dennis Lavrov
 Page: Tree of Life
Porifera. Sponges.
Authored by
Dennis Lavrov.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
Page: Tree of Life
Porifera. Sponges.
Authored by
Dennis Lavrov.
The TEXT of this page is licensed under the
Creative Commons Attribution-NonCommercial License - Version 3.0. Note that images and other media
featured on this page are each governed by their own license, and they may or may not be available
for reuse. Click on an image or a media link to access the media data window, which provides the
relevant licensing information. For the general terms and conditions of ToL material reuse and
redistribution, please see the Tree of Life Copyright
Policies.
- Content changed 30 March 2009
Citing this page:
Lavrov, Dennis. 2009. Porifera. Sponges. Version 30 March 2009 (under construction). http://tolweb.org/Porifera/2464/2009.03.30 in The Tree of Life Web Project, http://tolweb.org/











 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site